DFT
 @Sudheer Ganisetti
@Sudheer Ganisetti
Diffusion of Carbon in High Silicon Steel
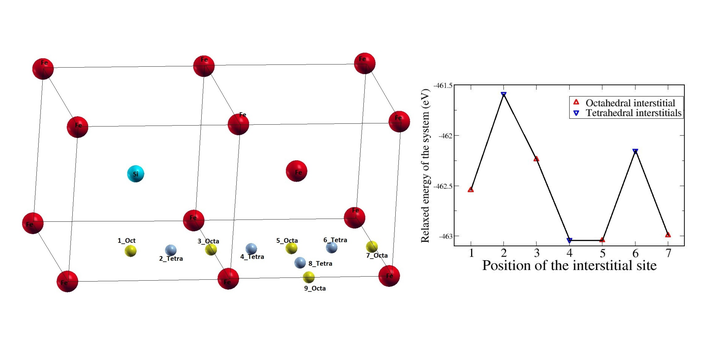
Many important phenomena in materials science involve diffusion of impurities. It is well known that diffusion of carbon in metals typically occurs via an interstitial mechanism, which can be strongly affected by the presence of other alloying elements. The interstitial diffusion of carbon controls the phase transformation in steel and therefore the resulting microstructure and mechanical properties. Density functional theory (DFT) based total energy and electronic structure calculations were performed with the Vienna Ab-Initio Simulation Package (VASP) to understand how silicon affects the the diffusion path of carbon in steel. The electron exchange and correlation interactions were approximated by the Generalized Gradient Approximation (GGA), and electron ion interactions were treated with Projector Augmented Wave (PAW) potentials. Through this work, we have studied two cases. In the first case, the energy barriers of one octahedral site to another octahedral site in the diffusion path of carbon atom away from the silicon atom in bcc Fe were computed, and these values were compared with the energy barriers of the pure bcc Fe system. In the second case, we have studied the possibilities of diffusion paths of C from octahedral position of one of the faces of a unit cell to the octahedral site of the neighboring face of the same unit cell.Fig (a) shows a supercell consisting of two unit cells. Silicon is substitutionally added to the body centred atom of the first unitcell, and carbon diffuses away from the silicon atom through interstitial positions 1_Oct, 2_Tetra, 3_Oc, etc., as shown in the fig (a). The fig (b) shows the total relaxed energy of the system of 53 Fe atoms with one substitutionally added Si and one interstitial C atom along the diffusing path of C from 1st octahedral site to the 7th Octahedral site away from the Si. After finding the formation energies and energy barriers, we conclude that the silicon traps the carbon atom to its second nearest neighbor tetrahedral position or third nearest neighbor octahedral position. It was also revealed that the diffusion path of C from Octahedral site of one face to the octahedral site of the neighboring face is possible only through the tetrahedral interstitial site even in the presence of Si.